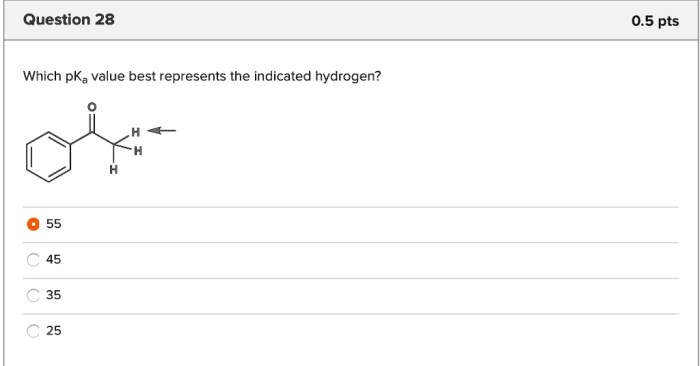
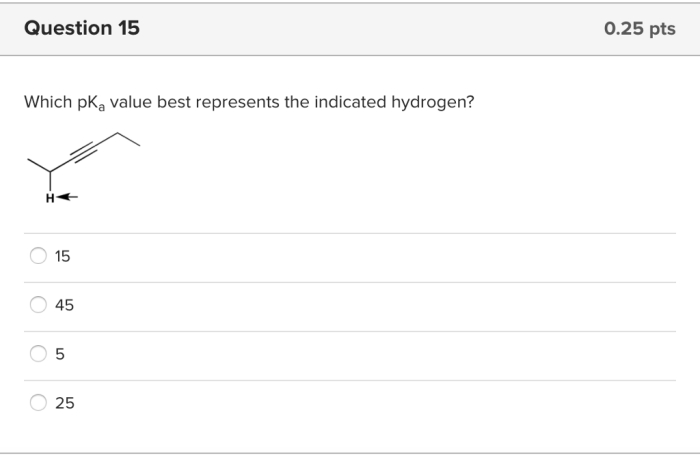
Which pka value best represents the indicated hydrogen – Understanding the pKa value of a hydrogen is crucial in chemistry. It provides insights into the acidity or basicity of a compound, influencing its behavior in various chemical and biological processes. This article delves into the concept of pKa, exploring the factors that affect it and its practical applications in diverse fields.
The pKa value of a hydrogen is a measure of its acidity, indicating the tendency of the hydrogen to dissociate from its parent molecule. A lower pKa value signifies a stronger acid, while a higher pKa value indicates a weaker acid.
Factors such as the hybridization of the carbon atom bonded to the hydrogen, the electronegativity of the atoms bonded to the hydrogen, and resonance effects can influence the pKa value.
Definition of pKa Value: Which Pka Value Best Represents The Indicated Hydrogen
The pKa value of a hydrogen atom refers to its tendency to dissociate as a proton (H+). It is a measure of the strength of an acid, with a lower pKa value indicating a stronger acid. The pKa value is calculated using the following equation:
pKa =
log(Ka)
where Ka is the acid dissociation constant.
Factors Influencing pKa Value
Hybridization of the Carbon Atom Bonded to the Hydrogen, Which pka value best represents the indicated hydrogen
The hybridization of the carbon atom bonded to the hydrogen affects the pKa value. sp3-hybridized carbon atoms have a higher pKa value than sp2-hybridized carbon atoms, which in turn have a higher pKa value than sp-hybridized carbon atoms.
Electronegativity of the Atoms Bonded to the Hydrogen
The electronegativity of the atoms bonded to the hydrogen also affects the pKa value. More electronegative atoms withdraw electron density from the hydrogen atom, making it more acidic and lowering the pKa value.
Resonance Effects
Resonance effects can also influence the pKa value. If the hydrogen atom is involved in resonance, the pKa value can be lowered due to the delocalization of the negative charge.
pKa Values of Common Functional Groups
| Functional Group | pKa Value |
|---|---|
| Carboxylic acids | ~4 |
| Alcohols | ~15-18 |
| Amines | ~9-11 |
| Amides | ~15-17 |
Applications of pKa Values
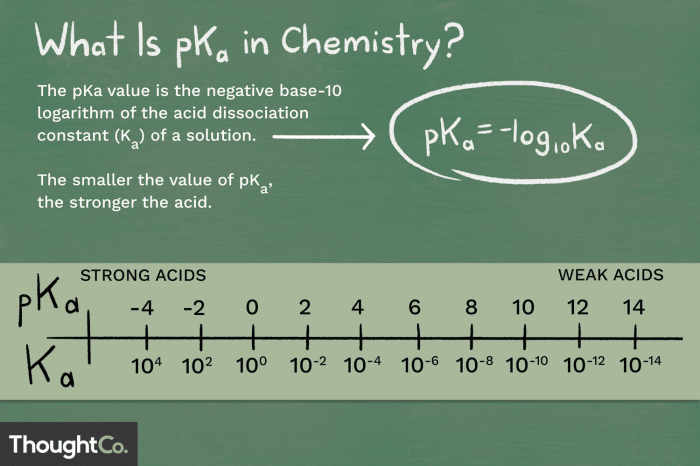
Acid-Base Titrations
pKa values are used in acid-base titrations to determine the equivalence point, which is the point at which the moles of acid are equal to the moles of base.
Buffer Solutions
pKa values are used to design buffer solutions, which are solutions that resist changes in pH. Buffer solutions are important in many biological and chemical applications.
Drug Design
pKa values are used in drug design to optimize the absorption, distribution, metabolism, and excretion (ADME) properties of drugs.
Experimental Determination of pKa Values
Potentiometric Titrations
Potentiometric titrations are a common method for determining pKa values. In this method, the pH of a solution is measured as a function of the amount of acid or base added.
Spectrophotometric Methods
Spectrophotometric methods can also be used to determine pKa values. These methods rely on the fact that the absorption spectrum of a compound changes as its pH changes.
Limitations of pKa Values
pKa values are useful for predicting the acidity or basicity of a compound in water. However, it is important to note that pKa values can change in different solvents or under non-standard conditions.
FAQ Overview
What is the significance of pKa values in chemistry?
pKa values provide a quantitative measure of the acidity or basicity of a hydrogen, helping to predict the behavior of compounds in chemical reactions and biological systems.
How can the pKa value of a hydrogen be experimentally determined?
Potentiometric titrations and spectrophotometric methods are commonly used to experimentally determine the pKa value of a hydrogen.
What factors influence the pKa value of a hydrogen?
The hybridization of the carbon atom bonded to the hydrogen, the electronegativity of the atoms bonded to the hydrogen, and resonance effects can all influence the pKa value of a hydrogen.